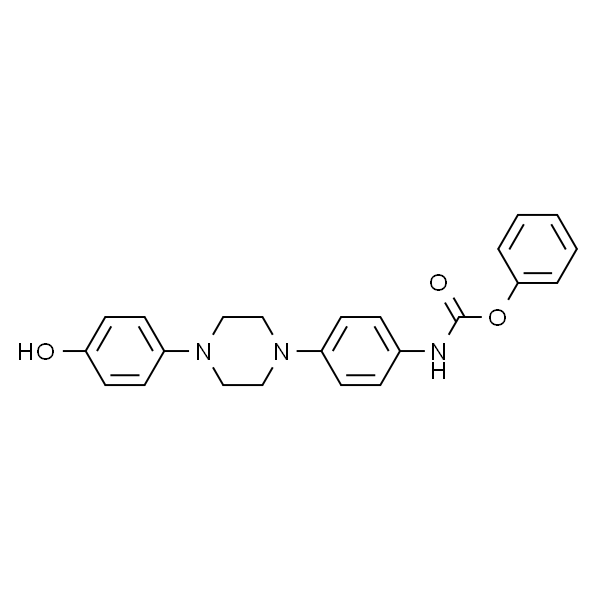
Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate CAS 184177-81-9 Posaconazole Intermediate High Quality
Factory Supply with High Purity and Stable Quality
Posaconazole Related Intermediates Commercial Production
Posaconazole CAS 171228-49-2
Posaconazole Intermediate POA CAS 149809-43-8
Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate CAS 184177-81-9
1-(4-Aminophenyl)-4-(4-hydroxyphenyl)piperazine CAS 74853-08-0
2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxal CAS 170985-85-0
Diethyl L-(+)-Tartrate CAS 87-91-2
| Chemical Name | Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate |
| Synonyms | Posaconazole Intermediate |
| CAS Number | 184177-81-9 |
| CAT Number | RF-PI290 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C23H23N3O3 |
| Molecular Weight | 389.45 |
| Density | 1.296 |
| Shipping Condition | Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Pale Brown to Grey Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Heavy Metals | ≤20ppm |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Posaconazole (CAS 171228-49-2) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate (CAS 184177-81-9) is an Intermediate of Posaconazole (CAS 171228-49-2). Posaconazole is a broad-spectrum, second generation, triazole compound with antifungal activity. It was approved for medical use in the United States in September 2006, and is available as a generic medication. Posaconazole is a triazole antifungal drug marketed in the United States by Schering-Plough under the trade name Noxafil. Posaconazole works by disrupting the close packing of acyl chains of phospholipids, impairing the functions of certain membrane-bound enzyme systems such as ATPase and enzymes of the electron transport system, thus inhibiting growth of the fungi. It does this by blocking the synthesis of ergosterol by inhibiting of the enzyme lanosterol 14a-demethylase and accumulation of methylated sterol precursors.
-
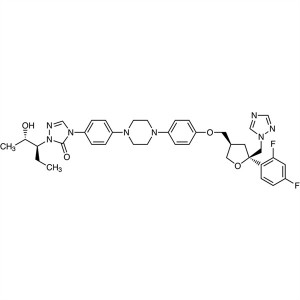
Posaconazole CAS 171228-49-2 API Factory Triazo...
-
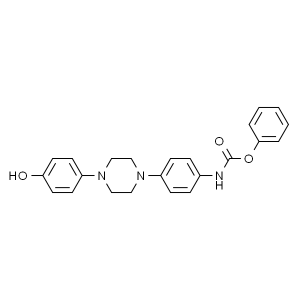
Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)ph...
-
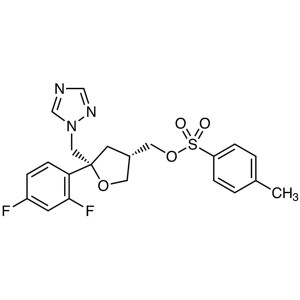
Posaconazole Intermediate POA CAS 149809-43-8 F...
-
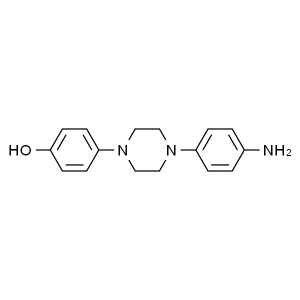
1-(4-Aminophenyl)-4-(4-hydroxyphenyl)piperazine...
-
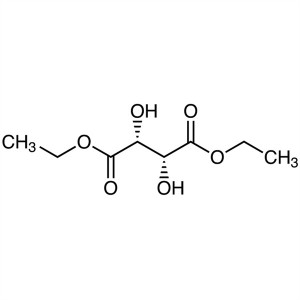
Diethyl L-(+)-Tartrate CAS 87-91-2 Purity ≥99.0...
-
![Posaconazole Intermediate CAS 170985-85-0 2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](https://www.ruifuchem.com/uploads/Posaconazole-Intermediate-CAS-170985-85-0-N-2S3S-2-Benzyloxypentan-3-ylformohydrazide-High-Quality-300x300.png)
Posaconazole Intermediate CAS 170985-85-0 2-[(1...