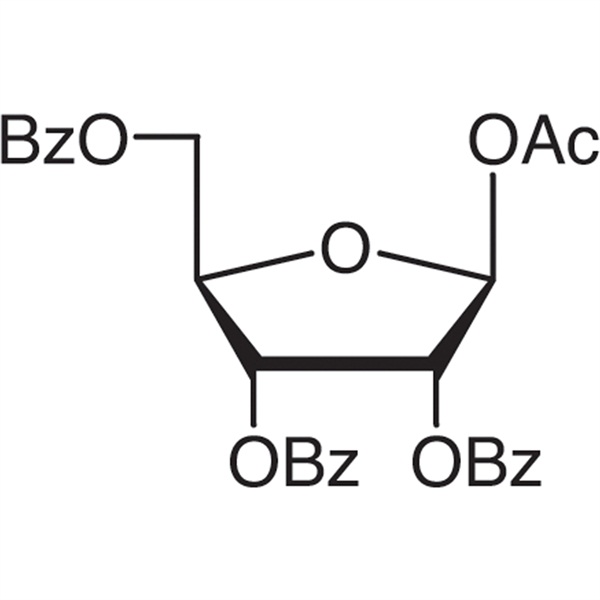
β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate CAS 6974-32-9 Assay ≥99.0% (HPLC) Clofarabine Intermediate High Purity
Commercial Supply Clofarabine Related Intermediates:
Clofarabine CAS: 123318-82-1
2-Deoxy-2-fluoro-1,3,5-tri-O-benzoyl-α-D-arabinofuranose CAS: 97614-43-2
β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate CAS: 6974-32-9
1,3,5-Tri-O-benzoyl-D-Ribofuranose CAS: 22224-41-5
2,6-Dichloropurine CAS: 5451-40-1
| Chemical Name | β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate |
| Synonyms | 1-O-Acetyl-2,3,5-tri-O-benzoyl-β-D-Ribofuranose |
| CAS Number | 6974-32-9 |
| CAT Number | RF-PI222 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C28H24O9 |
| Molecular Weight | 504.49 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| Assay / Analysis Method | ≥99.0% (HPLC) |
| Melting Point | 126.0~133.0℃ |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Total Impurities | ≤1.00% |
| Heavy Metals | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediate of Clofarabine CAS: 123318-82-1 |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate (CAS 6974-32-9) is an intermediate of (Clofarabine CAS: 123318-82-1). Clofarabine (CAS: 123318-82-1) is a novel purine nucleoside anticancer drugs is first successfully developed by the Top10 biopharmaceutical company of the United States-Genzyme Corporation with the trade names being “Clofarabine”. On December 28, 2004 the US food and Drug Administration (FDA) used fast-track for approval of clofarabine for application to children with refractory or relapsed acute lymphocytic leukemia (ALL); it has an excellent efficacy on the treatment of leukemia with well tolerance and no unpredictable adverse reactions. It can be administrated through either administered intravenously or administered orally. This drug is the first product approved for being dedicated to the treatment of the children's leukemia in more than ten years.
-
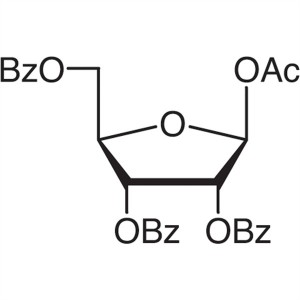
β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate CA...
-
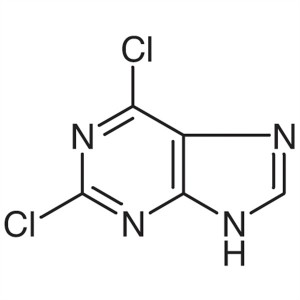
2,6-Dichloropurine CAS 5451-40-1 Assay ≥99.0% (...
-
D-(-)-Ribose CAS 50-69-1 Assay 97.0~102.0% Fact...
-
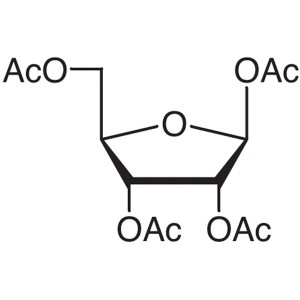
β-D-Ribofuranose 1,2,3,5-Tetraacetate CAS 13035...
-
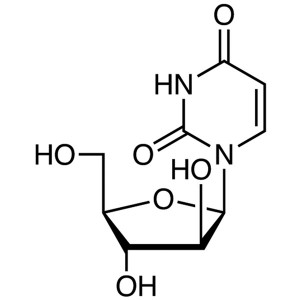
Arabinofuranosyluracil (Ara-U) CAS 3083-77-0 As...
-
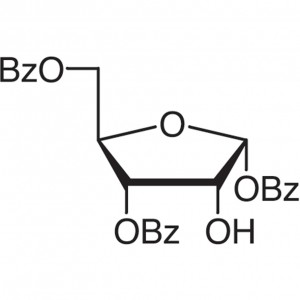
1,3,5-Tri-O-benzoyl-D-Ribofuranose CAS 22224-41...
-
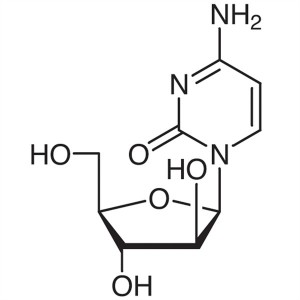
Cytarabine (Ara-C) CAS 147-94-4 Assay 98.0%~102...
-
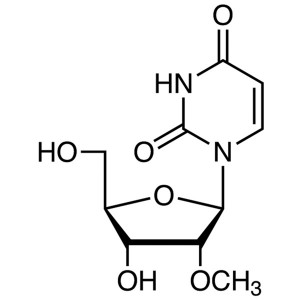
2′-O-Methyluridine CAS 2140-76-3 Purity ≥...
-
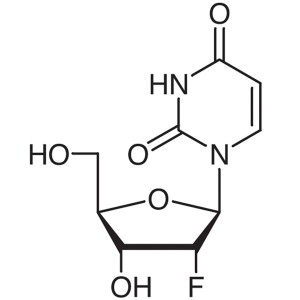
2′-Deoxy-2′-Fluorouridine CAS 784-7...
-
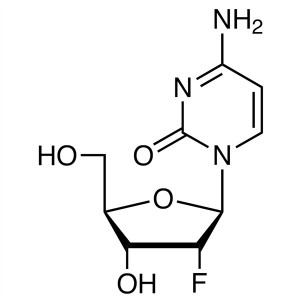
2′-Deoxy-2′-Fluorocytidine CAS 1021...